Notice that the proton closest to the carbonyl group is at a higher chemical shift than the proton in cyclohexene 6 05 ppm for cyclohexenone vs.
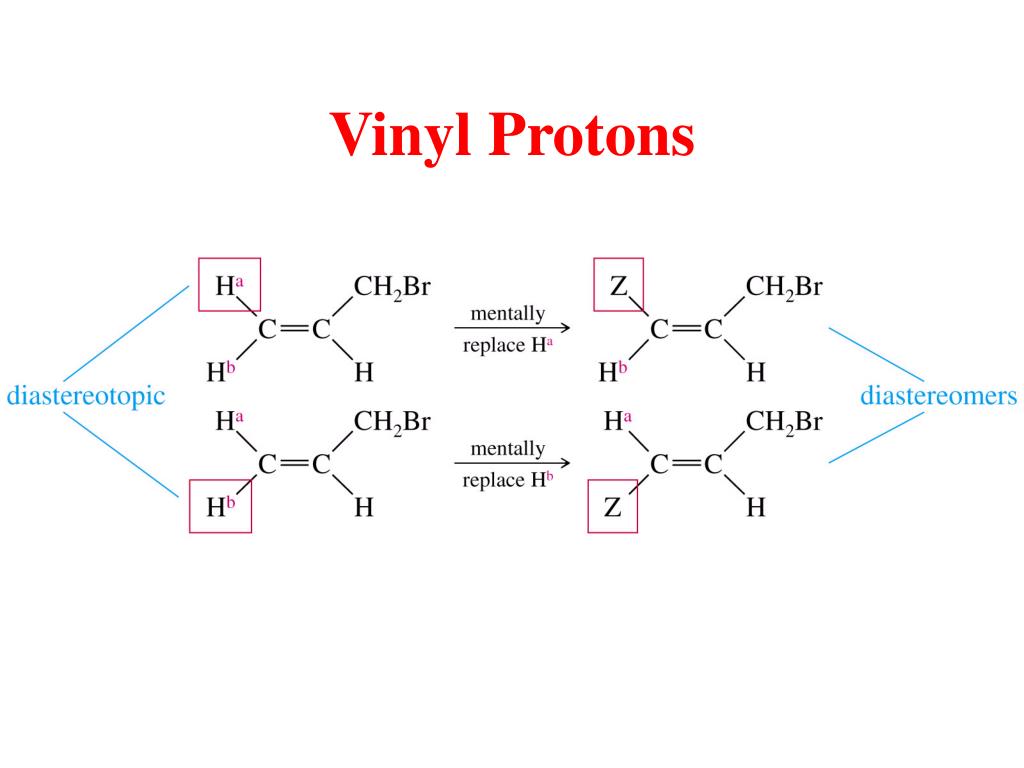
Vinylic protons nmr.
0 at the vinylic protons.
This means that a greater frequency is required to bring them into reso nance eq.
Consequently their nmr absorptions occur at relatively high chemical shift.
The source of spin spin coupling.
Table of characteristic proton nmr shifts type of proton type of compound chemical shift range ppm rch 3 1 aliphatic 0 9 r 2 ch 2 2 aliphatic 1 3 r 3 ch 3 aliphatic 1 5 c c h vinylic 4 6 5 9 c c h vinylic conjugated 5 5 7 5 c c h acetylenic 2 3 ar h aromatic 6 8 5 ar c h benzylic 2 2 3 c c ch 3 allylic 1 7 hc f.
Chemical shift is associated with the larmor frequency of a nuclear spin to its chemical environment.
The 1 h nmr spectra that we have seen so far of methyl acetate and para xylene are somewhat unusual in the sense that in both of these molecules each set of protons generates a single nmr signal.
In fact the 1 h nmr spectra of most organic molecules contain proton signals that are split into two or more sub peaks.
In other words frequencies for chemicals are measured for a 1 h or 13 c nucleus of a sample from the 1 h or 13 c resonance of tms.
The induced field therefore augments the local field at the vinylic protons.
As a result the vinylic protons are subjected to a greater local field.
This is not surprising since the proton is not only vinylic but it is also alpha to a carbonyl group.
Proton nuclear magnetic resonance proton nmr hydrogen 1 nmr or 1 h nmr is the application of nuclear magnetic resonance in nmr spectroscopy with respect to hydrogen 1 nuclei within the molecules of a substance in order to determine the structure of its molecules.
Tetramethylsilan tms ch 3 4 si is generally used for standard to determine chemical shift of compounds.