About 80 of production involves suspension polymerization.
Vinyl chloride polyvinyl chloride structure.
Vinyl chloride is primarily used to make polyvinyl chloride to manufacture plastics.
It is insoluble in alcohol and slightly soluble in tetrehydrofuran.
Vinyl chloride is an organochloride with the formula h 2 c chcl that is also called vinyl chloride monomer vcm or chloroethene this colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride pvc.
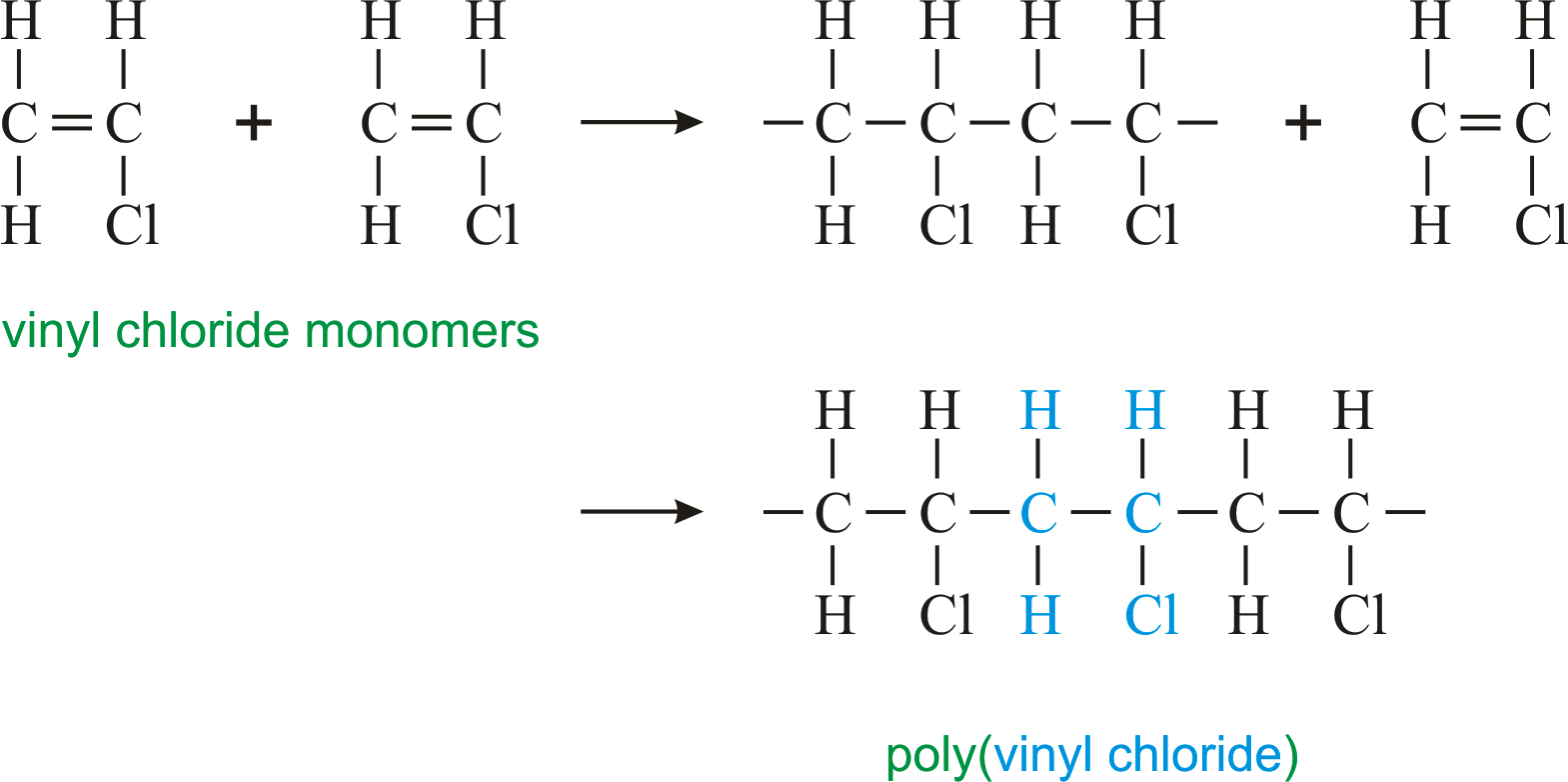
Polyvinyl chloride structure 2 pvc is a white brittle solid available in powder form or granules formed through an addition polymerisation reaction between vinyl chloride monomers figure 1.
Vinyl chloride is a chlorinated hydrocarbon occurring as a colorless highly flammable gas with a mild sweet odor that may emit toxic fumes of carbon dioxide carbon monoxide hydrogen chloride and phosgene when heated to decomposition.
This polymerisation reaction proceeds by a free radical mechanism.
Polyvinyl chloride is produced in an addition polymerisation reaction using the chloroethene vinyl chloride monomer.
Polyvinyl chloride is a white brittle solid.
Pvc is the world s third most widely produced synthetic plastic polymer after polyethylene and polypropylene about 40 million tons of pvc are produced each year.
Polyvinyl chloride was synthesized by baumann in the 1870s.
Effect of hydrogenation and chlorination reactions on the β relaxation of poly vinyl chloride journal of macromolecular science part b.
About 13 billion kilograms are produced annually.
Polyvinyl chloride is a white rigid quite brittle solid.
Oldring in encyclopedia of physical science and technology third edition 2003.
This solid form can then be modified with the addition of fillers and plasticisers depending on the task at hand.
Additives are used to modify the properties of polyvinyl chloride to make it more useful.
Second only to pe in production and consumption pvc is manufactured by bulk solution suspension and emulsion polymerization of vinyl chloride monomer using free radical initiators vinyl chloride ch 2 chcl is most often obtained by reacting ethylene with oxygen and hydrogen chloride over a copper catalyst it is a carcinogenic gas that must be handled with.
Xiv a vinyl chloride containing polymers.
Vinyl chloride is an organohalogen compound that has important industrial applications.
Pvc is used in the manufacture of numerous products including packaging films and water pipes.
However because of its insolubility and intractability it remained a laboratory curiosity until it was plasticized by waldo semon in the 1930s.
When treated with certain catalysts vinyl chloride monomers undergo polymerization and form the larger compound known as polyvinyl chloride or pvc.